|
Table 2: Pharmacogenomic predictors of drug hypersensitivity reactions in people with HIV infection |
||||||
|
Drug |
Phenotype |
HLA |
Population |
PPV |
NNP |
NNT |
|
Nevirapine |
SJS/TEN |
HLA-C*04:01 ? CYP2B6 983 T-C |
African (Malawian) Mozambique |
ND |
||
|
Rash |
HLA-B*35:05 HLA-Cw4 |
Thai, African, Asian, European White, Black, Asian Han, Chinese |
16% |
97% |
ND |
|
|
DRESS |
HLA-B*14/Cw8 HLA-Cw*8 or HLA -B*35 HLA -B*35:05 HLA -B*35:01 CYP2B6 516 G-T+C*04 CYP2B6 (rs2054675, rs3786547, rs3745274) |
Italian Japanese Asian Asian (Thai) European/Australian White, Black, Asian White, Black, Asian |
ND |
|||
|
Delayed rash (non-specific) |
B*35:05; RS1576*G CCHR1 status (GWAS) |
Thai |
||||
|
Hepatitis |
HLA-DRB1*01:01 (CD% > 25) and DRB1*01:02 |
Australian, European, South African |
18% |
96% |
||
|
Abacavir |
Hypersensitivity syndrome |
HLA-B*57:01 |
European, African |
55% |
100% |
13 |
|
Dapsone |
Rash, hepatitis |
HLA-B*13:01 |
Chinese |
7.8% |
99.8% |
84 |
|
Efavirenz |
Delayed rash (non-specific) |
DRB1*01 |
French |
ND |
||
|
Raltegravir |
DRESS |
HLA-B*53:01 |
African/Hispanic |
ND |
||
|
Sulfamethoxazole |
SJS/TEN |
HLA-B*38 |
European |
ND |
||
|
INR = international normalised ratio. * Indications for assessment by a liver transplant centre include Child–Pugh score ≥ B7, MELD score ≥ 13 or one of the following clinical events: refractory ascites, spontaneous bacterial peritonitis, hepatorenal syndrome, recurrent or chronic hepatic encephalopathy, small hepatocellular carcinoma or severe malnutrition. |
||||||
Figure 1: Approach to sulfonamide allergy in HIV-infected patients requiring trimethoprim sulfamethoxazole prophylaxis
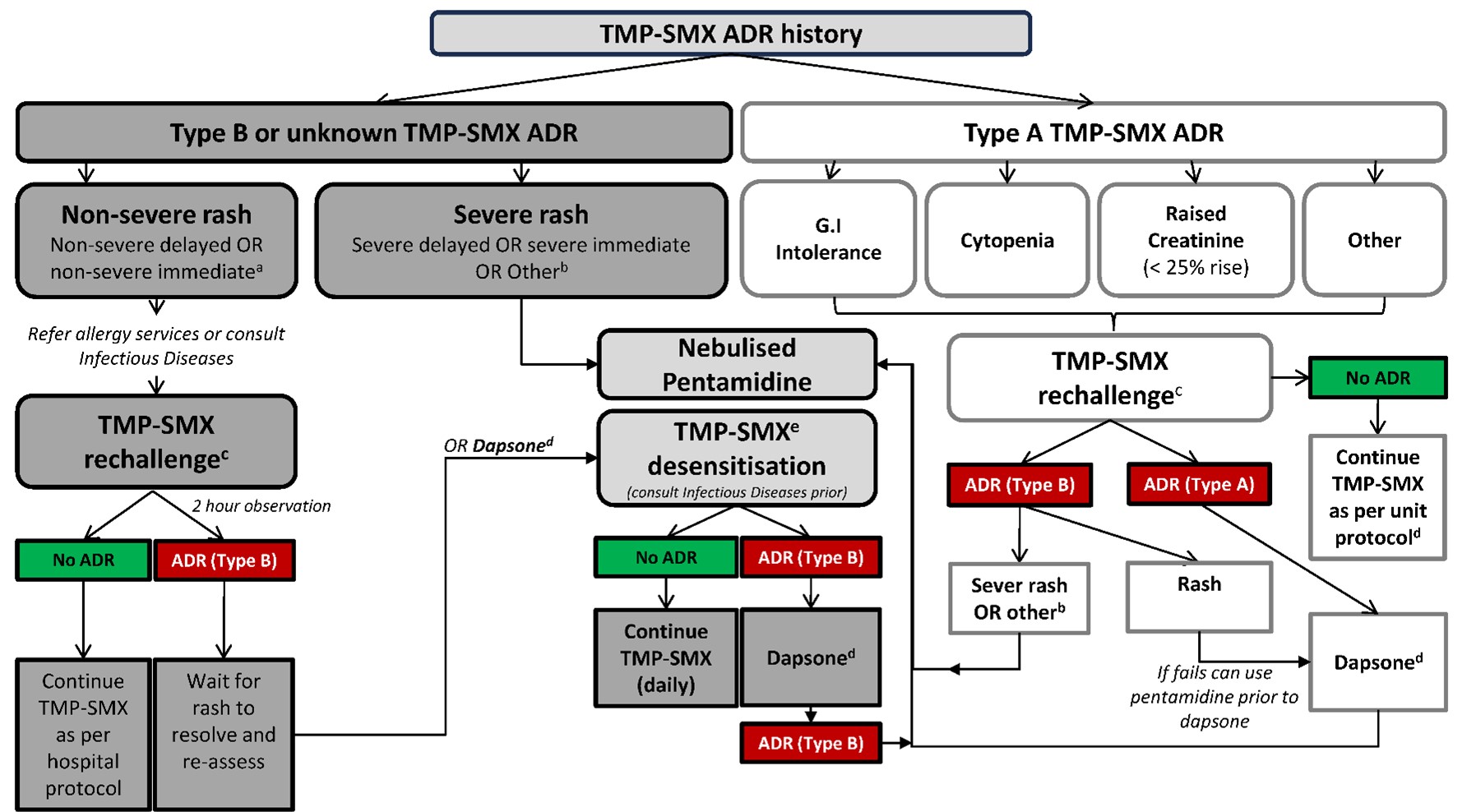
Legend: Type A – Non-immune-mediated adverse drug reactions Type B – Immune-mediated adverse drug reaction. For definitions of severe and non-severe immediate and delayed reactions see “Antimicrobial Hypersensitivity” Chapter of Australian Therapeutic Guidelines (v. 16).96
a If TMP-SMX-associated rash within last five years, can consider dapsone rather than rechallenge
b Drug fever, acute interstitial nephritis, fixed drug eruption, severe rash with mucosal ulceration or blistering/desquamation.
c Oral single dose challenge and observe for two hours (TMP-SMX 80mg-400mg) for non-severe delayed reactions. For historical non-severe immediate reactions (> 5 years) can consider an observed two-step TMP-SMX challenge (8mg-40mg orally then 72mg-360mg 30mins post initial) and observe 2 hours.
d Prescribe dapsone 100mg orally daily. Ensure G6PD deficiency screen negative prior to use.
e For all patients proceed with TMP-SMX desensitization or alternatively, dapsone therapy may be employed.
Adapted from 41,45,52,96

