In addition to those conditions mentioned above, data suggest a role for antiretroviral drugs in adding to the risk of CKD in patients with HIV infection45. The risk of CKD relates not only to the use of potentially nephrotoxic antiretroviral drugs but also to the patient’s comorbid conditions, polypharmacy and advancing age, as well as to HIV-specific factors37,46. Some antiretroviral drugs, in particular, may place a patient at increased risk of CKD, as shown in Table 337. In addition, some antiretroviral drugs may increase the serum creatinine level because of their effect on the excretion of creatinine by the proximal renal tubule, as seen in Table 3, causing an apparent change in glomerular function, without any real change in the patient’s actual GFR.
|
Table 3. Renal effects of current antiretroviral therapy (adapted from Swanepoel et al.37). |
|
|
Antiretroviral agent |
Possible renal effects |
|
Tenofovir disoproxil fumarate |
Renal dysfunction (acute or chronic) Proximal tubular dysfunction Fanconi syndrome (rare) |
|
Ritonavir/lopinavir |
Chronic kidney disease |
|
Ritonavir/darunavir |
Crystalluria |
|
Ritonavir/atazanavir |
Inhibition of tubular creatinine excretion Renal dysfunction (acute or chronic) Crystalluria |
|
Cobicistat (with elvitegravir, tenofovir disoproxil fumarate, emtricitabine) |
Inhibition of tubular creatinine excretion |
|
Cobicistat/atazanavir (with elvitegravir, tenofovir disoproxil fumarate, emtricitabine) |
Inhibition of tubular creatinine excretion |
|
Dolutegravir |
Inhibition of tubular creatinine excretion |
|
Rilpivirine |
Inhibition of tubular creatinine excretion |
TDF is a widely prescribed and a very effective antiretroviral drug that is excreted by the kidneys. Dose adjustments are required in patients with significant CKD47. TDF may also be prescribed for the prevention of HIV infection in people who are at high risk of HIV acquisition37. TDF may cause acute renal impairment, CKD and proximal renal tubular dysfunction. TDF is found at high concentrations in the proximal renal tubular cells and can cause mitochondrial toxicity. In some cases, the tubular dysfunction may be subclinical but in some patients it may be associated with the Fanconi syndrome; a clinical triad consisting of proteinuria, hypophosphataemia and renal glycosuria48. If the Fanconi syndrome is seen in patients on TDF, the drug should be ceased, as these effects may be significant, and in some cases life-threatening. Subclinical proximal tubular dysfunction may also be clinically important for long-term renal function, as well as bone health. In this situation there are limited data to suggest a uniform clinical approach to these adverse effects and dose reduction, increased vigilance with monitoring, or cessation of TDF may be appropriate. Overall, serious adverse renal effects are uncommon with TDF; however, because of the potential clinical importance of these effects, increased vigilance with screening for nephrotoxicity is suggested, as shown in Figure 430,37. Around 1-2% of all patients on TDF will need to stop treatment because of tubulopathy.
The newer formulation of tenofovir (TFV), tenofovir alafenamide (TAF), is metabolised differently to TDF and demonstrates increased intracellular levels of TFV in lymphocytes, but lower levels in the plasma and proximal renal tubules. Studies suggest much less renal toxicity with this new formulation49. Longer-term safety data for this novel agent are emerging. No specific screening tests are recommended in addition to the usual health checks in HIV-infected patients receiving TAF. HIV-infected patients treated with TAF remain at high risk of CKD and should be screened regularly for renal risk factors, and for the presence of CKD. There is also some evidence to suggest that TAF is effective for HIV pre-exposure prophylaxis, and it may also have a superior side-effect profile for this purpose, when compared to TDF50.
Figure 4. A suggested approach for renal monitoring in patients treated with potentially nephrotoxic antiretroviral agents, and agents which may artefactually affect the serum creatinine (Reproduced with permission, from Holt et al.30).
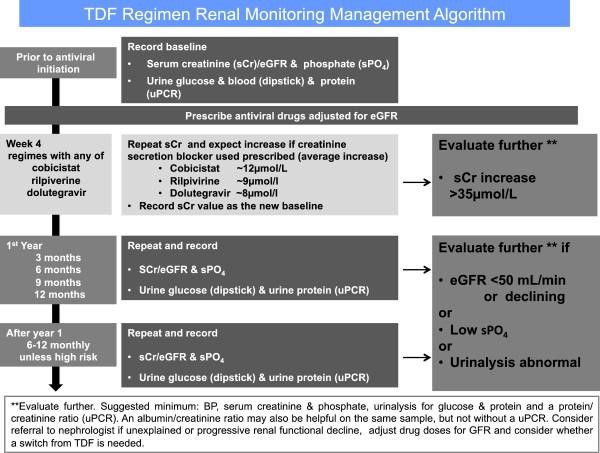
Some antiretroviral drugs are recognised to inhibit the excretion of serum creatinine by proximal renal tubular cells by the blockade of specific transporters. These agents; cobicistat (COBI), dolutegravir (DTG) and rilpiverine (RPV), act on different transporters in the proximal tubular cell, but are not directly toxic to the cell itself45. These drugs may increase the serum creatinine level, without altering the patient’s glomerular function. The actual GFR is unchanged; however, the serum creatinine level is artificially increased, with a resultant apparent reduction in the eGFR37. This effect is seen because serum creatinine is not an ideal measure of glomerular function; it is both filtered by the glomerulus (80-90%), as well as excreted by the proximal tubular cell (10-20%). The increase in the serum creatinine level seen after starting these agents occurs early, usually within 2-4 weeks, and is not progressive29. The increase in eGFR should not usually exceed 25% in patients with normal renal function but may be higher in those patients with pre-existing CKD. The evaluation of renal impairment and the calculation of drug doses may become challenging in patients treated with these agents, particularly those with established CKD37. The detection of TDF-related nephrotoxicity may also be an issue and tubular function should be closely monitored, as shown in Figure 429,30,37.
Polypharmacy and the risks of drug-drug interactions are high in HIV-infected patients, particularly in the older patient group51. This is relevant given the ageing of HIV-infected patients and the associated increasing burden of NICMs, requiring treatment with additional non-ART medications. Polypharmacy is also associated with adverse drug reactions and drug-drug interactions and with poor treatment adherence. Drug dosing and interactions are important considerations, particularly in HIV-infected patients with CKD52. Medication issues are much more frequently seen in HIV-infected patients, when compared to the uninfected population. In recent times there has been a move to simpler two-drug ART regimens in an attempt to simplify therapy and improve tolerability53.

