As detailed above, the clinical presentation of PHI is non-specific, and not easily distinguishable from other viral illnesses on clinical grounds alone. It is therefore critical that the diagnosis is considered in all people with a compatible clinical profile, especially those who have a high risk of acquiring HIV infection. Rapid, efficient early diagnosis is a critical step in the treatment cascade aimed at preserving both the individual’s health and immune competence and preventing onward transmission of the infection. Usually, the confirmation or exclusion of the diagnosis of PHI in the laboratory is relatively straightforward, therefore the critical step in the process is the consideration of the diagnosis as a possibility.
Principles of laboratory testing for primary HIV infection
Traditionally, the mainstay of laboratory diagnosis of HIV infection relies on the demonstration of serum antibodies to the virus following infection. These antibodies are produced after several weeks of infection. There is therefore a ‘window period’ following infection when antibody tests will remain negative, potentially leading to false negative diagnostic tests in the setting of PHI. The length of this period is variable and depends on the target for detection by a particular assay; for example, tests capable of detection of all anti-HIV antibodies including anti-HIV IgM have a shorter window period than those that detect anti-HIV IgG alone. There is also a period referred to as the ‘eclipse period’, which lasts approximately 10 days wherein there is no diagnostic test capable of detecting HIV26 (Figure 2). The first marker detectable following the eclipse period is viral nucleic acid, which is detected by a nucleic acid test (NAT), also known as nucleic acid amplification test (NAAT) (Figure 2). Approximately 50% of infected patients will have detectable HIV-1 DNA or RNA within 12 days of infection27. The HIV-1 capsid protein p24 antigen is the next marker of infection able to be detected from around day 15, and is often cleared by day 50, making it a useful marker of PHI28. By day 20, anti-HIV IgM antibodies are able to be detected, with IgG emerging shortly after and persisting throughout the course of infection28 (Figure2).
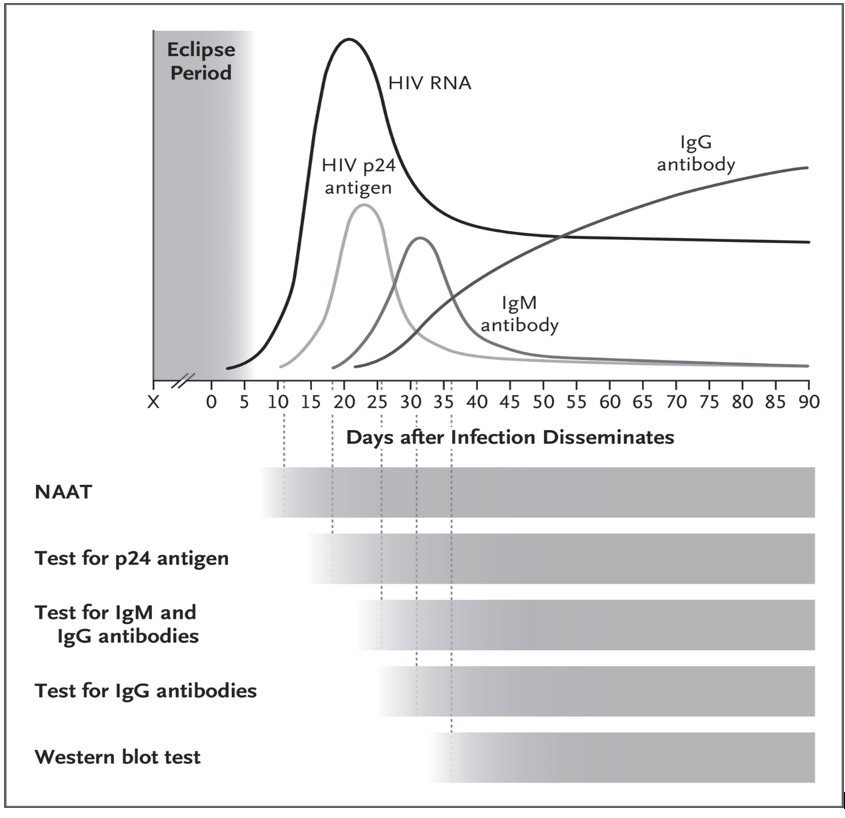
Figure 2. Progression of HIV Viremia and Immune Response after Initial Infection. Reprinted from "HIV Infection - Screening, Diagnosis, and Treatment". Saag MS, 2021, N Engl J Med;384(22):2131-43. It is important to understand the impact of pre-exposure prophylaxis (PrEP) on the window period; users of PrEP who become infected have been shown to have delayed seroconversion on both laboratory-based and point-of care (POC) tests29, 30, so a high index of suspicion should remain in those with known or suspected poor adherence. In Australia, most laboratories utilise 4th generation HIV antibody/antigen screening tests, with which time to detection is at most 3-4 weeks from infection, with rare outliers. Point-of-care testing is available in some jurisdictions; however, it is important to note that these tests have substantially longer window periods to detect infection in comparison to laboratory-based tests and therefore have the possibility of delivering false negative tests if conducted shortly after HIV infection.
Serum antibody tests
Since 1985, there have been four generations of antibody tests for HIV, with each improving upon its predecessor in terms of sensitivity and shortening the window period. These utilise an enzyme immunoassay (EIA) in which native or synthetic HIV antigens, fixed on a solid phase, are exposed to and bound by HIV antibodies in test serum. These antibodies are then detected by a second antibody to human IgG for detection by the assay. These tests are rapid, low cost, and have a sensitivity greater than 99.5%31, making them the standard screening tool for HIV infection. First and second-generation assays are referred to as ‘IgG sensitive’ as they detect only anti-HIV IgG from patient specimens. So-called third generation tests are IgM/IgG sensitive tests and function to shorten the window period to the earliest point of IgM detection, reported to be a median of 23 days after infection27. Fourth-generation tests, known as antigen/antibody (Ag/Ab) combination tests utilise an antibody test paired with simultaneous p24 antigen detection. In this manner, the window period can be shortened to just 18 days after infection27.
Although false positive EIA tests are rare (specificity is reported to be greater than 99.8%), positive tests should be followed-up with a confirmatory test. Typically, this will be a HIV-1/2 confirmatory assay, which is a rapid laboratory-based test which confirms a positive fourth-generation Ab/Ag assay and differentiates HIV-1 antibodies from HIV-2 antibodies32. The Western Blot is the preferred confirmatory test prior to the HIV-1/2 confirmatory assay. It involves detection of antibodies in patient sera that react with the various HIV proteins, which have been separated into bands based on their distinct molecular weight, using protein gel electrophoresis and transferred (blotted) onto a nitrocellulose membrane. Antibodies to these proteins arise in a well-defined and predictable order (Figure 3). First, antibodies to the structural group-specific antigen (Gag) proteins, p24 and its precursor p55 appear, usually within 2 weeks. Next arise the antibodies to the envelope glycoproteins – the precursor gp160, the extracellular gp120, and the transmembrane gp41; these persist throughout all stages of infection. Last to become detectable are antibodies to the polymerase gene products p31, p51 and p66. By three months following infection, the Western Blot will be fully evolved33, 34. This knowledge can be used to help define early sero-converters and provide clues regarding time-course of infection.
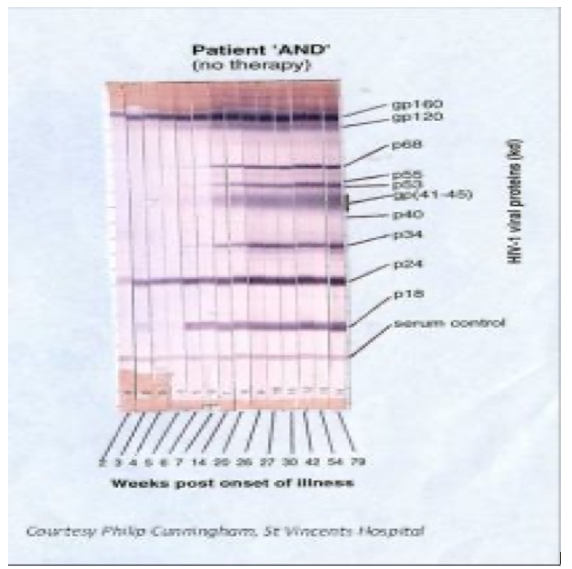
Figure 3. Typical appearance of an evolving HIV-1 Western Blot in HIV infection (seroconversion). Reprinted from NSW State Reference Laboratory for HIV, St Vincent’s Hospital Sydney Limited Indeterminate Western Blots should be followed by repeat serology after 4 to 6 weeks to evaluate for an evolving antibody response. It is currently not possible to determine the time of initial infection through the use of laboratory tests alone, except in the very early phase of infection through the use of an evolving Western Blot antibody pattern in conjunction with supplementary assays, such as detection of p24 antigen and HIV RNA or DNA (Figures 2 & 3).
Detection of virus components
Detection of viral components in plasma, serum or blood leucocytes is useful in the diagnosis of early HIV infection, given that virus is detectable in the blood before the generation of detectable antibodies, typically by day 10 following infection. This usually shortens the window period, especially in patients with negative or indeterminate serological testing. The most commonly used assays test for viral nucleic acids (RNA in plasma or proviral DNA in blood leucocytes) or the p24 antigen (in serum).
P24 antigen detection
The p24 antigen is detected via an EIA and is a highly specific diagnostic test in the setting of PHI and able to detect up to 90% of infected individuals seeking testing. It is, however, only positive for a short period of time post infection and is not sensitive in chronic HIV infection 35, 36 (Figure 2). The p24 antigen test is now most commonly performed as part of the fourth-generation antibody/antigen test.
Nucleic acid testing
Being the first detectable marker of PHI, plasma HIV-1 nucleic acid testing via polymerase chain reaction (PCR) is highly sensitive and specific in the diagnosis of PHI. It is a very useful adjunct in patients with negative or indeterminate antibody tests. Quantitative real-time PCR for viral RNA (RT-PCR) is primarily used for the purpose of monitoring responses to ART in established HIV infection, however it can be used in primary infection for diagnostic purposes with caution. False positive tests are possible, though decrease in likelihood with increasing HIV viral load. Therefore, the test should be repeated at least once if the viral load is low (<1000 copies/mL)37, 38. When used in this manner, the window period for NATs is slightly shorter than that of the p24 antigen. NATs remain positive throughout untreated HIV infection (Figure 2). Detection of proviral DNA in blood leucocytes can be used for the early diagnosis of HIV infection in babies infected through mother to child transmission. Passive transfer of maternal antibody to the infants in utero renders antibody testing problematic, conferring a high rate of false positive results for up to 6 months after birth.

