Immune-based therapies were initially trialled as alternatives or adjuvants to cART, particularly in patients with CD4+ T cell depletion. Subsequently immune modulators have been used to reduce the immune activation associated with HIV infection and, in particular, gut bacterial translocation. Most recent work has focused on the use of immune modulators as a mechanism to facilitate the clearance of HIV in cure strategies.
Latent HIV infection
After suppression of HIV replication and viraemia with cART, there is persistent reservoir of latent HIV infection, predominantly within memory CD4+ T cells (Chomont et al., 2009) and to a limited extent in myeloid cells including, monocytes and dendritic cells in the gut. (Cattin et al., 2019) This CD4+ T cell reservoir is likely to be maintained by replication of the infected cells rather than low level HIV replication. (McManus et al., 2019) The reservoir of HIV has been difficult to accurately measure as much of the integrated virus is defective and not all the intact virus can be activated by T cell activation or proliferation (Bruner et al., 2019)
Since latency represents the major barrier to eradication of HIV infection, strategies to activate HIV transcription in latently infected T cells and increase anti-HIV immune responses are under investigation. (Sengupta and Siliciano, 2018) This strategy designated ‘kick and kill’ involves activation (‘kick’) of HIV replication and the subsequent ‘kill’ of infected cells by a viral cytopathic effect or immune-mediated killing. (Deeks, 2012) Most of the initial work has involved the application in clinical trials of latency reversing agents (LRA) that could activate virus expression from latency in vitro.
Latency reversing agents
Early clinical trials using inhibitors of histone deactylase that could activate HIV from latency in vitro, such as sodium valproate (Lehrman et al., 2006), did not show any effect and subsequent work with more potent inhibitors, such as vorinostat,(Archin et al., 2012; Elliott et al., 2014) panabinostat (Rasmussen et al., 2014) and romidepsin (Wei et al., 2014) has shown that virus replication can be demonstrated in patients on therapy but that this replication is not associated with a significant increase in plasma HIV viral load or decrease in the size of viral reservoirs. More recent strategies have used an expanded range of different LRAs (Figure 10) and preclinical work has also used modulators of apoptosis to increase the virally mediated or immune mediated killing of HIV-infected cells. (Kim et al., 2018)
Figure 10. Latency reversing agents in development and clinical trials
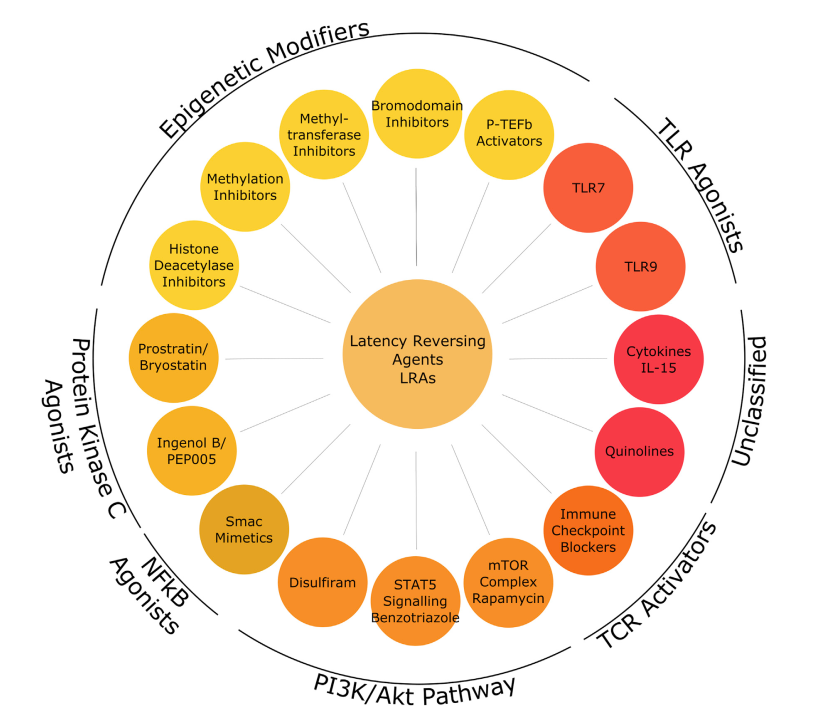
Note: The classes of compound acting to increase HIV expression from latency are shown in circles within the different mechanistic classes. All are classified here according the pathway of mediating increased HIV expression. A number of the classes also have an immunological effect and activate CTL (IL-15, Immune checkpoint blockers), or innate immunity (TLR7, TLR9).
P-TEFb = positive transcription elongation factor b; TLR = Toll-like receptor; mTOR = mechanistic target of rapamycin; STAT5 = signal transducer and activator of transcription 5; IL-15 = interleukin-15.
Source: Kim Y, Anderson JL, Lewin SR. Getting the “Kill” into “Shock and Kill”: Strategies to Eliminate Latent HIV. Cell Host & Microbe. 2018 Jan 10;23(1):14–26. Used with permission
Lack of effective killing may reflect changes of CTL killing in the presence of HDACi (Deng et al., 2015) or lack of activity of the current immune-based therapies.(Bullen et al., 2014) The range of LRAs and their combinations has expanded rapidly with the development of in vitro and ex vivo models of latency that can be used to test the efficacy of these agents in virus reactivation. However, the gold standard remains cells isolated from virally suppressed HIV infected individuals.(Sengupta and Siliciano, 2018) Use of cytokines, such as type I IFNs, IL-2, IL-7, IL-15 may potentially lead to reduction in reservoir size but larger definitive studies are still needed. Particularly promising is the use of TLR agonists that activate innate immunity leading to decreased viral DNA and viral reservoirs (Winckelmann et al., 2013; Tsai et al., 2017; Offersen et al., 2016)
Boosting anti-HIV immune responses
Specific stimulation of CTL to improve killing of HIV-infected cells has focused on the use of therapeutic vaccination, including DC vaccines, (García et al., 2013) and the use of cytokines, such as IL-7 and IL-15, alone or concurrently with therapeutic vaccines. The use of immune checkpoint blockers such as antibodies to CTLA-4 and PD-1 that can restore antiviral immunity is now being evaluated in clinical trials. Boosting immune responses with immune checkpoint inhibitors has been shown to be effective in cancer immunotherapy (Kim et al., 2018). Finally, CTLs can be engineered by introducing TCRs specific for HIV to produce chimeric antigen receptor-T cells (CAR-T cells). A more versatile and likely effective approach uses T cells engineered to recognize the T cell by an HIV-specific antibody, which avoids the need to individualize the CTL TCR for specific MHC recognition. These convertible CAR-T cells (cCAR-T cells) bind to the target cells by a modified monoclonal antibody that crosslinks the cytotoxic T cell to the HIV infected cell (Herzig et al., 2019)
Summary
Despite the efficacy of cART, immune-based therapies may have a complimentary role for both immune reconstitution and HIV cure. These interventions may augment CD4+ T cell numbers and cell-mediated immunity in the presence of viral suppression with cART. While the clinical benefits of immune-based therapy remain to be determined, augmentation of antibody-mediated control through potent neutralisation and ADCC, as well as T cell-mediated immune responses through use of immunomodulators, such as immune checkpoint blockers and CAR-T cells, may play a role to limit viral replication, reduce the risk of drug resistance and eradicate HIV reservoirs in people taking cART.

