Disturbances in glucose metabolism, such as insulin resistance, impaired fasting glucose, impaired glucose tolerance and diabetes mellitus, are amongst the most common endocrine disorders found in treated HIV infection (1, 2). Diabetes and its prediabetic disorders of impaired fasting glucose and impaired glucose tolerance are defined using the American Diabetes Association criteria (3), using either fasting glucose, random glucose or HbA1c or glucose responses to glucose tolerance test, as shown in Box 1. Diabetes may be preceded by a prodrome of either impaired fasting glucose or impaired glucose tolerance which may be present for some 5-10 years prior to progression to overt diabetes mellitus. Detecting prediabetes is a golden opportunity for diabetes prevention, as will be discussed below. Insulin resistance is difficult to measure in individual patients and is not recommended. Measures such as fasting insulin levels or insulin levels following an oral glucose load are unreliable and accurately not-reproducible (4).
Box 1. Criteria for diagnosis of diabetes mellitus and other glucose disorders (American Diabetes Association).
|
Fasting glucose1 |
2 hour glucose2 |
Random glucose |
HbA1c3 |
|
|
Normal |
< 5.6 mmol/L |
|||
|
Diabetes mellitus |
> 7.0 mmol/L |
> 11.1 mmol/L |
> 11.1 mmol/L with symptoms4 |
> 6.5% |
|
Impaired fasting glucose |
5.6 - 6.9 mmol/L |
|||
|
Impaired glucose tolerance |
7.8 - 11.0 mmol/L |
1: fasting is defined as no caloric intake for at least 8 hours.
2: following 75g of an anhydrous glucose load, dissolved in water.
3: HbA1c: glycated haemoglobin, measured on non-fasted blood.
4: polyuria, polydipsia or unexplained weight loss.
Prior to the availability of cART, most diabetes cases occurred in patients treated with pentamidine for Pneumocystis jirovecii infection and were characterised by rapid onset of insulin deficiency and ketoacidosis (type 1 diabetes mellitus; T1D), since pentamidine destroys pancreatic insulin-secreting beta-cells (5-7). The most common form of diabetes mellitus in HIV-infected patients in the cART era is type 2 diabetes (T2D), due to a combination of insulin resistance and insufficient insulin secretion to meet demand (1).
The exact prevalence and incidence of diabetes in treated HIV infection has been difficult to establish precisely and is affected by a number of factors, including exposures to different cART medications such as HIV nucleoside reverse transcriptase inhibitors (NRTIs) and HIV protease inhibitors, in addition to different prevalence rates in promoters of diabetes such as obesity, high-energy diets and sedentariness. Further, there are clear ethnic groups with high genetic susceptibility to diabetes. Prevalence rates for T2D with the use of older cART regimens (to which many surviving patients were exposed) range from 7%–13% (8-10). More recent studies show diabetes rates in HIV patients to be twice that of age-matched controls (11), with rates of impaired fasting glucose and diabetes of 9.1% and 4.5% respectively in an Italian cohort (12). The Swiss HIV Cohort reported diabetes rates of 7% (13), higher than the 3% rate found in the Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D) Study (14). Incidence studies report 5.72 cases /1000 patient-years of follow-up in the D:A:D Study after 3.8 years follow-up (14) and 14.1 cases/1000 patient-years after 10-years follow-up in a large French study (15). Recent observational data on diabetes in US youth with HIV-infection show a 5-fold rate increase in the periods of time between 2004-7 and 2008-2014 (16). This may reflect the impact of the US obesity epidemic in promotion of diabetes, differences in ascertainment and diabetes screening over the observation period or differences in cART used over time.
The longest longitudinal study of diabetes incidence has been undertaken in our cohort followed at St Vincent’s Hospital for up to 18 years, where we recently reported incident rates of prediabetes at 33% and incident diabetes at 13% (17), according with other international studies of shorter duration (18-25). In accord with US data where obesity has likely impacted diabetes incidence, Australian data showed that a modest gain of 250-330g of central abdominal fat in mostly healthy weight patients was associated with a 3-fold increase risk of diabetes incidence (17). The USA Veterans Aging Cohort Study reported that every 2.3kg of weight gained in the 12 months after cART initiation, increased the risk of diabetes by14% (26). The D:A:D cohort study found that every 1.0 kg/m2 BMI gain was associated with a 12% increased risk of incident diabetes over the 12 months after cART initiation, with a 2.6-fold increased risk in those in the highest quartile of BMI gain regardless of the pre-cART BMI category (27).Together, these studies show that even modest weight gain in cART recipients increases the risk of metabolic comorbidities and suggest that efforts should be aimed at preventing weight gain in healthy weight people living with HIV infection or adressing overweight or obesity.
The treatment of T2D in people with HIV infection requires lifestyle changes with an emphasis on healthy weight and, more importantly, healthy waist circumference, with appropriate nutritional and physical activity intervention. Since the epidemic of overweight and obesity now affects more than 50% of Australians, this has also affected people with HIV infection. Those at higher risk of T2D include people with a family history or ethnic susceptibility (eg people with Indigenous Australian, South East Asian, South Asian, Pacific Islander or South American Indian heritage), who need particular consideration for assessment of diabetes risk, diabetes screening for early detection, and early intervention.
In addition to weight-optimisation and physical activity, metformin is the first line glucose lowering medication. In people with treated HIV infection, metformin is an insulin sensitiser, reduces abdominal obesity, reduces hepatic glucose uptake and improves glyacemic control (28). Metformin is contraindicated in the presence of severe renal impairment or cardiac failure, where it may induce lactic acidosis. Care should be taken in patients receiving NRTIs, who have impaired renal function or elevated plasma lactic acid levels. Further, the integrase inhibitor dolutegravir can markedly increase circulating metformin levels. Metformin is eliminated by active renal tubular secretion through the organic cation transporter molecules, the action of which dolutegravir inhibits, thereby increasing post-dose metformin levels substantially. The maximal recommended metformin dose in people receiving dolutegravir is 1000mg daily. The risk of lactic acidosis is further increased where there is renal impairment and in states of dehydration. The clinical manifestations of hyperlactinaemia are non-specific. Diagnosis may be delayed by unpredictability in the onset of mitochondrial toxicity. Symptoms include abdominal pain, nausea, vomiting, weight loss, fatigue, dyspnoea, seizures, impaired cognition, arrhythmias and heart failure. Blood investigations may reveal anaemia and leukopaenia as well as elevation in aminotransferases, lipase and creatinine phosphokinase. All NRTIs may also cause hyperlactinaemia, however older drugs carry the highest risk (zidovudine, lamivduine, stavudine and didanosine) (29).
In a patient who develops lactic acidosis, the NRTI medication or dolutegravir should be immediately ceased. Supportive care is the mainstay of treatment, aiming to optimise tissue oxygen delivery by cardiopulmonary support. This includes fluid resuscitation and mechanical ventilation, if required. Resolution of hyperlactinaemia requires replenishment of mitochondrial DNA which may take 4-28 weeks to recover (29). After normalisation of lactate levels a new antiretroviral drug regimen should be considered, with non-nucleoside reverse-transcriptase inhibitors (NNRTI) and protease inhibitors being a safer regimen for these patients. In those patients on dolutegravir plus metformin, an alternative glucose-lowering medication should be considered.
Other glucose lowering medications available to use in T2D include oral (sulfonylureas, dipeptidyl peptidase IV inhibitors, sodium glucose co-transporter 2 inhibitors) and injectable (glucagon-like peptide-1 analogues, insulin) agents. An individualised therapeutic approach should be undertaken to bring glucose levels to target, using glycated haemoglobin (HbA1c) as an index of glucose levels over three months. HbA1c targets are individually set, based on age and the risk of adverse effects from hypoglycaemia. Usual HbA1c targets are 6.5-7.0% for most adults with T2D and 7.0-7.5% for adults with type 1 diabetes. Higher HbA1c targets are approproiate in the elderly, those with hypoglycaemia unawareness or with a history of severe past hypoglycaemic events.
In addition to glucose level control, standard diabetes care includes aggressive cardiovascular risk reduction (applying secondary prevention targets for fasting lipid levels and blood pressure), with annual review for diabetes complications, including examination for neuropathy, retinopathy and measurement of urinary microalbumin level. Despite these standards of diabetes care, there is evidence from both local and international studies of lower rates of achieving target glycaemic, lipid and blood pressure control and lower rates of complications screening in people living both with HIV infection and diabetes, compared to people without HIV infection (30-33). These data suggest there may be treatment disparities, perhaps related to clinical differences, greater clinical complexity or, perhaps, clinical inertia. As people living with HIV infection are enjoying near-normal life expectancy and are at greater risk of diabetes-related complications and cardiovascular disease, it is critical benchmarked standards of care are achieved. Strategies to support best practice may include focused diabetologist education on HIV infection, internal audits of diabetes care clinical targets and adherence to complication screening. Greater emphasis on nutrition therapy and physical activity in conjunction with pharmacotherapy may reduce the metabolic and cardiovascular burden. We have previously recommened embedding a specialised diabetologist within HIV specialist services as an opportunity to ensure the standards of care are met for people living with both HIV infection and diabetes (30), however this model of care has not been investigated for efficacy.
Prevention of diabetes is feasible in people with HIV infection. Many cases of T2D can be prevented by modest weight reduction, increased physical activity, a reduction in sedentariness and a diet that limits energy-dense foods. If prediabetes is detected, lifestyle management with modest weight reduction can prevent almost half progressing to diabetes mellitus. This can be augmented by the addition of metformin.
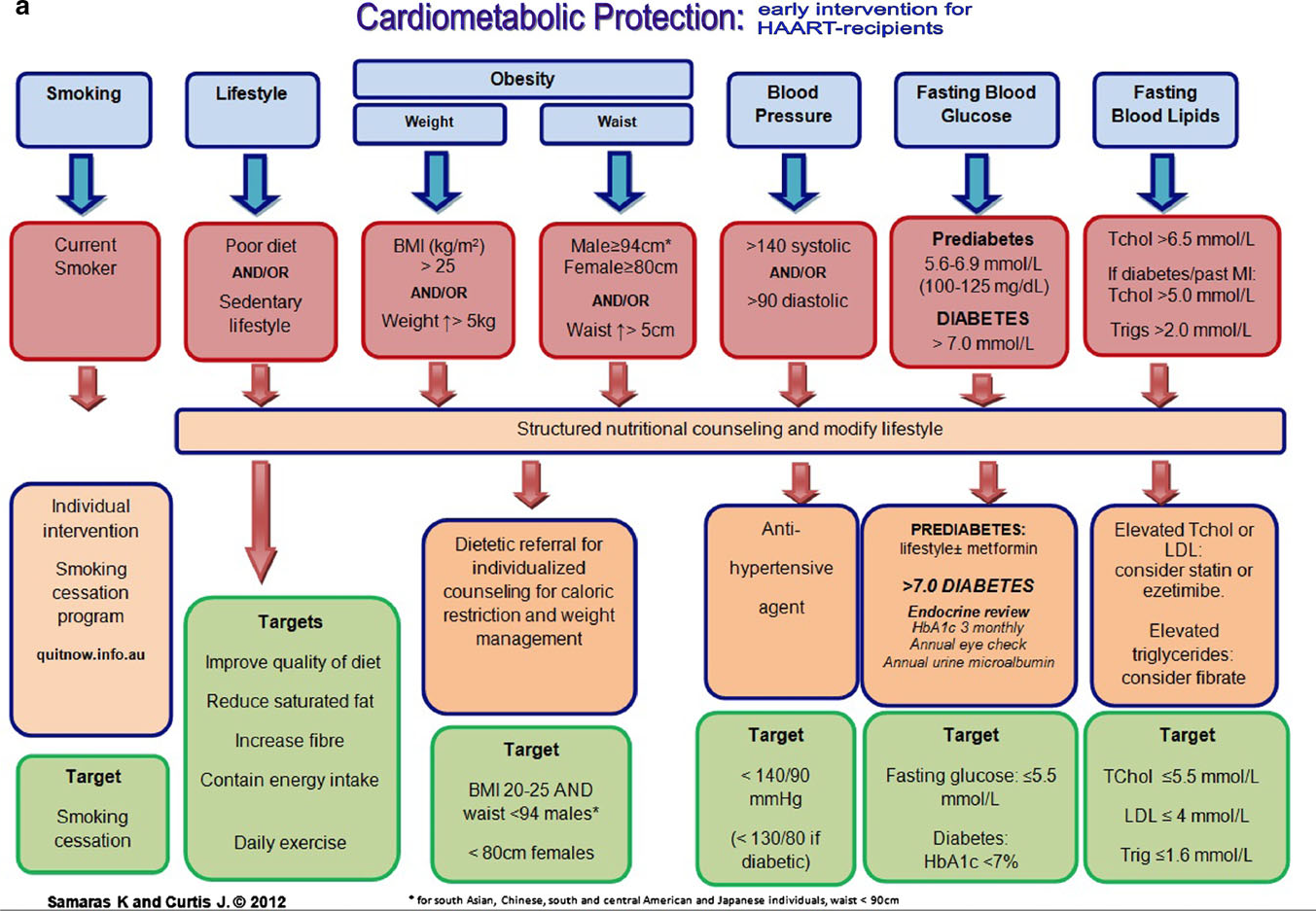
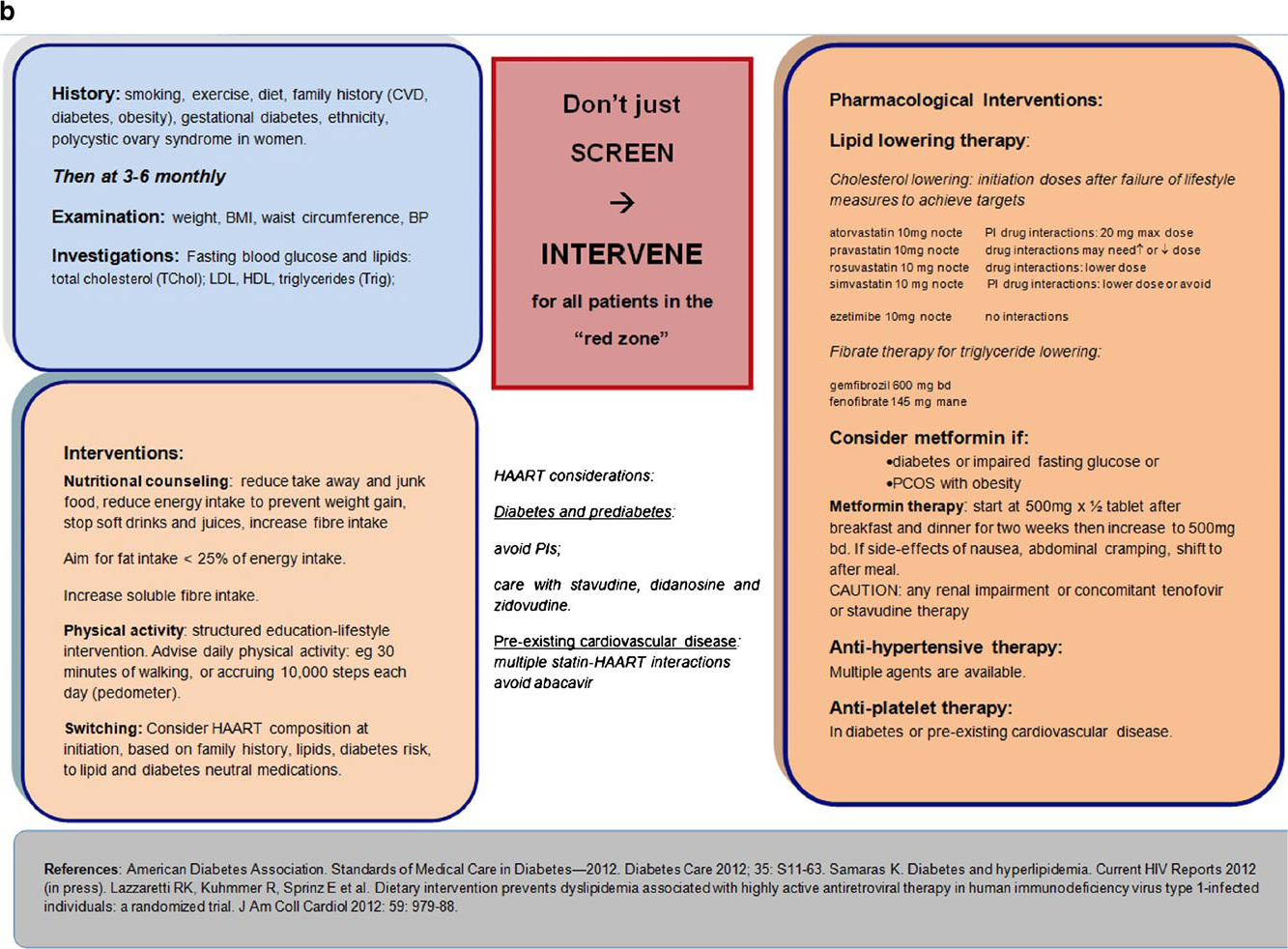
Figure 1 is a published cardiometabolic screening algoritm designed for the care of people with HIV, which may be useful in the primary and secondary setting in the cardiometabolic care of people with teated HIV infection.
Figure 1. Cardiometabolic screening algorithm designed for the care of people with HIV infection