HIVAN and HIV immune complex kidney (HIVICK) disease are the most frequent forms of renal disease directly related to HIV infection37. HIVAN is seen almost always in patients with advanced HIV infection and occurs predominantly in patients of African descent, explained by a genetic predisposition. Increased susceptibility to HIVAN among this group of patients is associated with polymorphisms in the APOL-1 gene7. HIVAN typically manifests clinically with heavy proteinuria, without haematuria. There is usually a rapid decline in renal function and increased echogenicity of the kidneys on renal ultrasound. Histologically, the lesion seen is a collapsing FSGS38. The mainstay of treatment for the underlying renal lesion is ART. Prednisone and antiproteinuric agents (ACE inhibitors, ARBs) may also be used39. Supportive treatments such as dialysis and renal transplantation may be required. The prognosis for patients with HIVAN remains poor but has improved following the widespread introduction of ART. Because of modern ART, HIVAN is now much less common among people with HIV infection. Indeed, non-collapsing FSGS is now more commonly seen than classic HIVAN at renal biopsy37. In these patients, plasma HIV RNA is usually undetectable and they are usually receiving ART. The histological findings may be difficult to distinguish from secondary FSGS in the general population40.
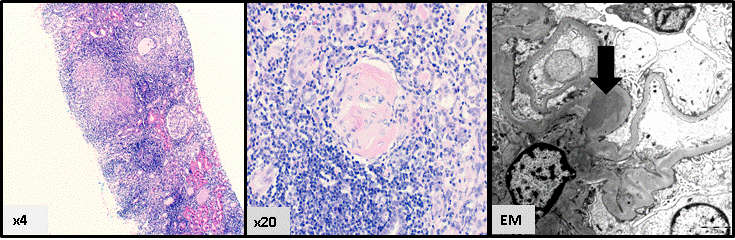
Numerous forms of immune complex kidney disease may also be seen in patients with HIV infection. Although the term HIVICK has been used to describe these conditions, a specific description of the pattern of immune complex deposition in the setting of HIV infection is preferred because of the lack of certainty regarding causality of the HIV infection and because of the heterogeneous nature of these conditions37. HIVICK is composed of a variety of histological conditions, including immunoglobulin A (IgA) disease, and a lupus-like syndrome41,42. It is characterised by the deposition of immune complexes within the kidneys, associated with cellular proliferation, inflammation and damage. Tubulointerstitial inflammation may be prominent, as shown in Figure 2. Clinically, these conditions have heterogeneous presentations; there may be microhaematuria, a variable degree of proteinuria and impairment of renal function. HIVICK benefits from ART and the prognosis appears to be more favourable when compared to HIVAN37. Other HIV-related renal diseases, such as thrombotic microangiopathy and diffuse infiltrative lymphocytosis syndrome (DILS) have been also reported but are not common37.
Figure 2. HIV immune complex kidney (HIVICK) disease, with prominent interstitial inflammation and glomerular hypercellularity (Figure 2a); glomerulosclerosis (Figure 2b); and deposits of IgA on electron microscopy (arrow) (Figure 2c) (Reproduced with permission of Dr Lyndal Anderson and Associate Professor Paul McKenzie, Tissue Pathology and Diagnostic Oncology, Royal Prince Alfred Hospital, Sydney NSW).
Other forms of renal disease
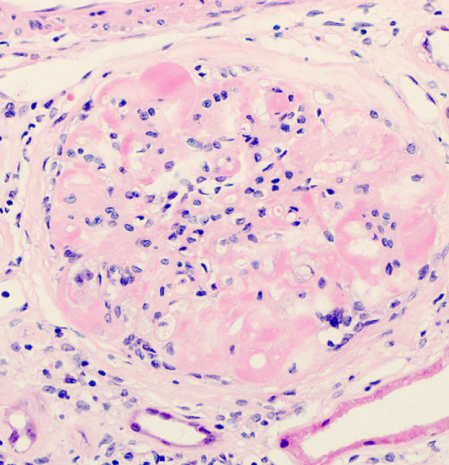
As in HIV seronegative people, the commonest causes of CKD in people with HIV infection are related to NICMs, in particular, hypertension and diabetes31. With the ageing of the HIV-patient cohort on ART and the increased burden of NICMs seen, renal disease related to these conditions is increasingly common43. The clinical and histological features of these conditions are similar to the general population, although some studies suggest an additional role for HIV infection in the progression of renal disease in this setting37. It can be difficult to separate the causation of non-classical FSGS in some patients; secondary FSGS may be related to hypertension, however, there may also be overlap with an HIV-related effect on the glomerulus37. Both conditions may be associated with proteinuria and CKD, although haematuria is less commonly seen. Their progression is usually insidious but may be abrogated by stringent attention to the patient’s vascular risk profile44. Diabetic nephropathy is characterised by nodular expansion of the glomerulus, with associated scarring and fibrosis, as shown in Figure 3. Hepatitis C virus co-infection may also increase the risk of CKD in HIV infection, particularly with a higher HCV viral load37.
Figure 3. Diabetic nephropathy in a patient with HIV infection. The glomerulus is expanded and exhibits nodular change (arrow) and patchy inflammation (Reproduced with permission of Dr Lyndal Anderson and Associate Professor Paul McKenzie, Tissue Pathology and Diagnostic Oncology, Royal Prince Alfred Hospital, Sydney NSW).